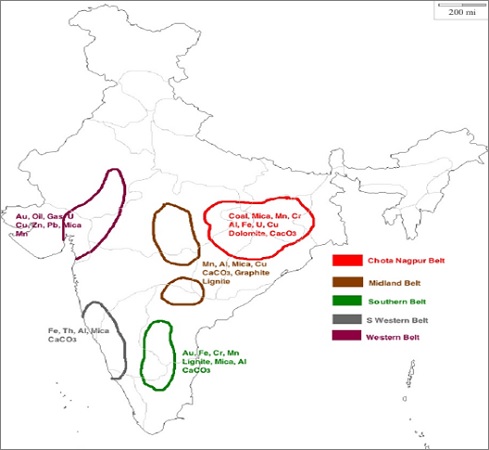
Mineral Resources India – Iron, Coal, Aluminium, Copper, Lead, Zinc
by Devender
0 2588
Mineral resources can be classified into two groups which are: Metallic and Non-Metallic.
The metallic minerals are further divided into Ferrous and Non-Ferrous.
- Ferrous - Contains Iron content (Iron ores, Ni, Co, Mn, etc.)
- Non-Ferrous - Gold, Silver, Cu, Pb, Al, Zn, etc.
- Organic - Fossil fuels such as Coal, Petroleum, etc.
- Inorganic - Mica, Limestone, Graphite, Gypsum, etc.
- Magnetite - Best quality iron ore containing 72 % iron
- Hematite - 60 to 70 % iron content
- Limonite - 40 to 60 % iron content
- Siderite - Nearly 40 % iron content
- iron ore, coking coal, and limestone
- water for cooling
- energy for heating
- Deciding locations for the iron-coal industry
- Wood supply was the primary factor for deciding the location
- Smelters were usually set up near forest areas
- Even in Modern times, Visvesvaraya Iron and Steel Plant (Karnataka) was set up near the jungle to get wood-charcoal
- Later it was switched to hydro-electricity from the Sarawati river
- The coalfield region had a tradition of ironworking based on charcoal
- Coalfield areas already had the labor and technology
- In Britain, iron ore was found embedded with coal seams
- During that era, to process 1 ton of iron ore 8-12 tons of coal was needed
- Railway engines were also inefficient
- So, weight-wise, it was cheaper to transport iron ore to coalfields rather than transporting coal to the iron ore site
- Need for Coking coal
- Jharkhand - Jamshedpur (TISCO), Bokaro
- West Bengal - Durgapur, Burnpur
- Odisha - Rourkela
- Chhattisgarh - Bhilai
- Andhra Pradesh - Vishakhapatnam
- Karnataka - Vishveshwarya
- Tamil Nadu - Salem
- During this phase, 50% or more weight loss happens
- This process is done near the raw material site
- Less transportation cost because of the less weight
- UP - Hindalco (Birla)
- Odisha - Hirakund (Birla), Jharsuguda (Vedanta)
- Chhattisgarh - Korba (Vedanta)
- BALCO - Ratnagiri, Maharashtra
- NALCO - Koratpur, Odisha
- MALCO - Mettur, TN
- Less weight means less transportation cost
- Therefore, copper concentrating mills are set up near the raw material (mines)
- Blister copper is immersed in a bath of copper sulfate
- Electricity is passed through it and impurities are removed
The weight loss ratio is extremely small and that is why there is no economic factor to set up copper refining factories near the raw material.
Copper Refineries of India
- Hindustan Copper - Khetri, Jhunjnu district, Rajasthan
- BACLO - Korba, Chhattisgarh
- Hindalco (Birla) - Dahej, Bharuch district of Gujarat
- Sterlite Industries - Tutikorin, Tamil Nadu
- The concentration stage involves significant weight loss so it is carried out near the mining site
- The refining stage requires a lot of electricity so it is done near large thermal plants/hydroelectric sites
- India doesn’t have sufficient ores of Lead/Zinc, so the majority of requirements met through imports
The Non-Metallic can be further divided into two groups i.e. Organic and Inorganic.
Mineral Resources India – Iron, Coal, Aluminium, Copper, Lead, Zinc
1 Iron & Coal Industries
Iron Ores
Iron-coal industry
Heating the Iron ore with coke & limestone gives Pig iron. Pig iron ore processing - cast-iron, wrought iron, steel, and a variety of alloys.
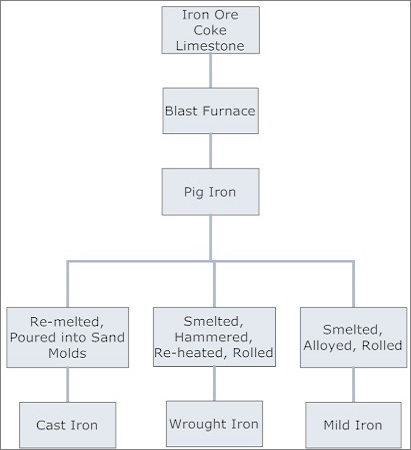
Essential inputs are:
The steel industry also requires dolomite, manganese, etc. but in small quantities. So, their presence is not the main deciding factor for the location.
Near Forest
Until the end of the medieval period, iron production was done on a small scale as energy was immobile, and to produce five tons of iron, you had to chop down one acre of forest to get sufficient charcoal.
Near coalfields
During the Industrial Revolution, the iron and steel industries were set up near coalmines for the following reasons:
Near coastal areas
By the early 20th century, coal and iron ore mines in US-Europe started getting depleted, so, they started importing iron ore from other countries. As a result, the iron space and steel industry started moving toward coastal sites to reduce the cost of transporting ores from port to factory via railways.
For Example - Steel plants at Vishakhapatnam, Ratnagiri, Mangalore
Iron ore has iron oxide but we only need the iron and thus need to get rid of the oxide. So, to remove the oxide part, we blend it with carbon to form Carbon dioxide. Coking coal has a high concentration of carbon, compared to cheap varieties of coal like Lignite. Therefore, it is used ot mix with iron ore.
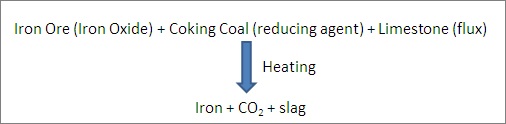
However, for heating, different varieties of coal and even electricity can be used.
2 Steel Industries India
3 Aluminium-Bauxite Refining
Bauxite to Alumina
Aluminum is an abundant mineral in the earth's crust but for mining or commercial scale exploitation, you require a significant concentration of bauxite ore at one particular site. The Bauxite occurs frequently in the tropical areas where limestone rocks are exposed to weathering.
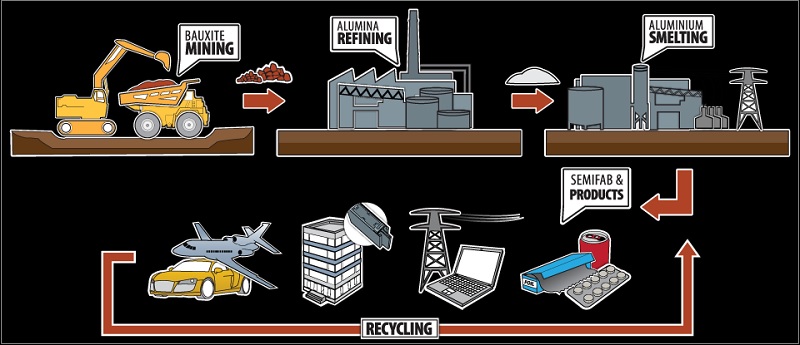
First, Bauxite ore is crushed, washed, and mixed with caustic soda to remove impurities, and then, it is dried in large furnaces to remove moisture content, and finally, you get alumina.
Alumina to aluminum
The white powder of alumina is dissolved in a bath and electric current is passed through it using carbon electrodes which convert alumina to aluminum.
It requires a massive amount of electricity that is why aluminum smelting facilities are set up near sources of cheap electricity, rather than near to raw material or near to final market.
Aluminium Industry of India
4 Copper Refining India
In the 20th century, copper became important for the electric industry and the demand for it increased. Hence, new mining-smelting technologies developed to utilize even lower quality ores.
The location principle for copper and aluminum industries same, but the smelting process is different.
Concentrating ore (Copper ore to Blister copper)
The Copper ore is soaked in water and mixed with oils. The copper revering matter floats on the top and is separated out for further processing. After this stage, only 2.5% of the original matter remains.
After this stage, sulfur and oxygen impurities are separated from the concentrated copper ore and blister copper is obtained. In this stage, the weight loss ratio is significant, therefore smelting is usually done near raw material.
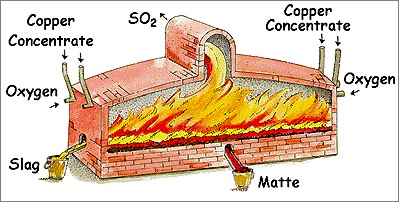
Blister to copper (Electrolysis)
Blister copper is 99% pure metal but still, it is not suitable for manufacturing electronic wires, utensils, etc. as it contains impurities of gold, silver, lead, and zinc. Therefore, blister copper is refined via the electrolysis method.
4 Lead and Zinc Industry
The ore goes through smelting and then refining through Electrolysis to get Lead and Zinc. It runs on the principle that certain minerals have an affinity for certain oils, therefore, the ore is mixed with water, oil, and chemicals.
The mineral particles get attach to oil bubbles and float on the surface, foam (containing mineral particles) is skimmed off.
Location Factors
| State | Ore | Smelter |
| Jharkhand | Lead | At Tundoo |
| Andhra Pradesh | Lead | Vishakhapatnam, based on imported lead concentrates |
| Rajasthan | Zinc | At Debari and Chanderia |
| Kerala | Zinc | Based on imported zinc concentrates |

Share:




Comments
Waiting for your comments